the unique attomarker platform
Technology Overview
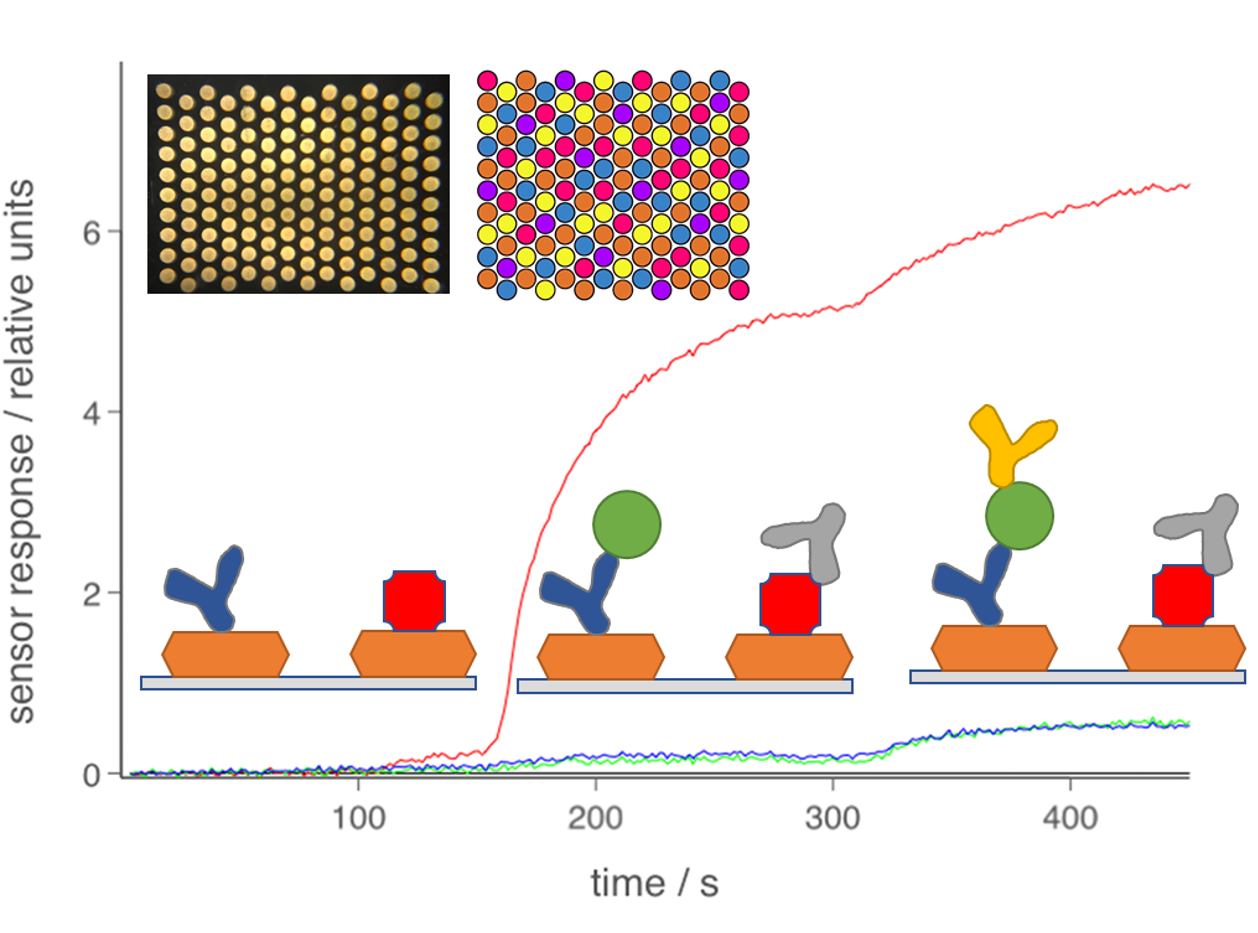
The core Attomarker technology is an array of gold nanoparticles which are printed into an array of sensors spots over which the blood sample flows. The spots are illuminated from below to produce scattered light that changes with the mass of biomarker on the surface of the gold nanoparticles, Figure 1. The mass sensing is unique because it allows a proper measurement to be made that is fully quantitative. This will set the new global standard for biomarker tests with all laboratories giving answers in standard units and the end to local standards.
The future of Attomarker is to develop the panels of tests that make a difference.
Liscar 6
The first production version of the technology evolved from the research laboratory in a hurry at the start of the pandemic and is the light scattering array reader (LISCAR) version 6, Figure 2. It is a bench-top instrument that can be used in clinics next to the patient at the point-of-care where the rapid test result can make a difference. It can also operate in a lab on a railway basis. It is easy to use and reports the results to the Cloud for quality control as soon as the measurement are made. A multi-use chip allows up to 20 tests to be performed on each sample and up to 48 different samples to be measured per day. The results are given to the clinician in time to change the diagnosis and treatment: 60% of blood tests arrive too late even in hospital. Our Attomarker Covid Antibody Immunity Spectrum test is now CA marked and will be rolled out through 2025.
A suite of different chips is currently in our rapid development pipeline including a Fatty Liver Test that looks at the state of the liver from healthy through the onset of type 2 diabetes to cirrhosis. In addition, a full profile of the hormones of the menstrual cycle will help us to understand properly fertility and hormone replacement therapy in the menopause.
The LISCAR 7 will include development for the ward and Intensive Care Units to take blood samples from the standard vacutainer blood collection method and prepare the blood before testing with an incorporated robot.
Attomarker is also currently developing a handheld version of the device with the same tests, targeting launch in late 2025.

Available late 2025
Handheld
The Attomarker technology can also work at home. A version of the instrument, still fully quantitative and able to measure many biomarkers from a drop of blood, is now completing development and will be in trials next year. The bench tops is replaced by a hand-held device, Figure 3, using an iPhone video camera to measure the brightness changes of the gold nanoparticles, calculate the result, and send it to the Cloud. Now we can provide laboratory-quality measurements, checked in the Cloud and passed to a clinician for a teleconsultation. The vision for the hand-held which will include a rapid infection chip to help manage sepsis. Pluse food allergy screening not just for IgE the component that causes allergic reaction, but also IgG that protects against allergy and rises as people become desensitised. We are also developingis to take high world-leading diagnosis to all parts of the globe.
The Human Cube
The technology is collecting high quality data in the Cloud which will populate the Human Cube, all fully anonymously, to look for patterns in human health, Figure 4. We all work in slightly different ways – endotypes – and we have already discovered some of these in the responses to the vaccine in the pandemic and in innate immunity. Even a small number of instrument sales will generate a huge data set and we hope to get to 1 billion data points in 5 years. The fleet of instruments will allow collection of the biggest most accurate data allowing us to perform the biggest clinical trial performed every day. The Human Cube will change the Science of Diagnosis.
