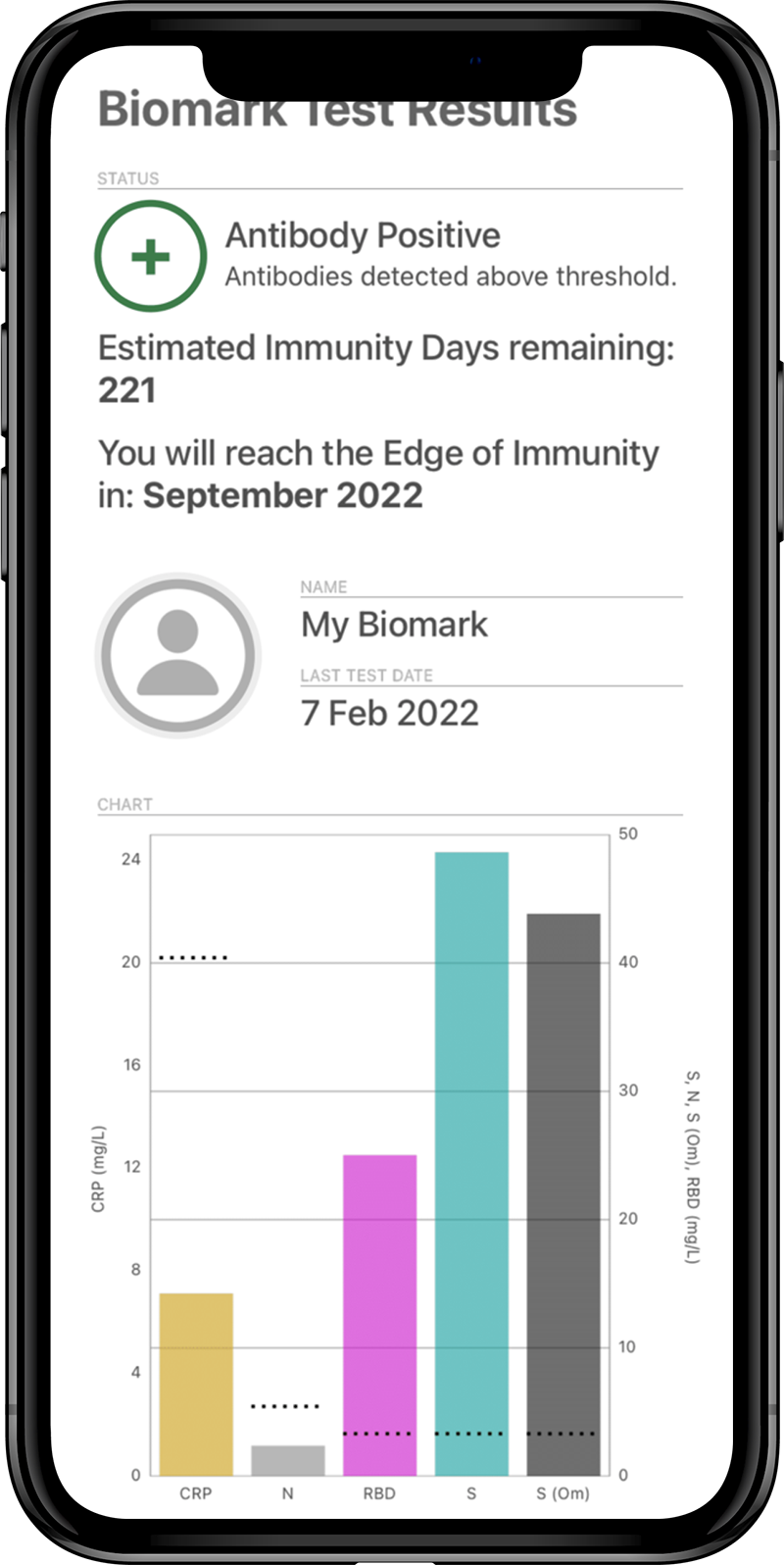
The Attomarker COVID19 Antibody Immunity test was CE marked and fully quantitative referenced to the NIST standard human antibody. The test measured the antibody immunity profile for antibodies binding to spike, omicron-spike, nucleocapsid and receptor binding domain proteins. The Antibody Immunity profile (Figure 5) provided the following information:
- Nucleocapsid (N) indicating past viremia;
- Spike Protein (S) indicating past infection or vaccination;
- Spike Protein from the Omicron Variant (SO) indicating vaccine binding to the Omicron variant or infection from Omicron;
- Receptor Binding Domain (RBD)
The test also measured the familiar C Reactive Protein. The Test was performed from venous blood with the results available in 7 minutes, presented as the Biomark (Figure 5) available on the iPhone app.
The concentration of S antibodies was used to assess the number of ‘Immunity Days’ to the ‘Edge of Immunity’ calculated from an antibody half-life of 60 days and a mucosal protection threshold of 3.4 mg/L, rounded to the nearest month.
The Edge of Immunity depended on antibody half-life and ranged from 60 – 200 days. The test could measure half-life more precisely; two samples 100 days apart were required as advised by clinicians.
The test was sold as part of a consultation in private healthcare to patients who believe they are immuno-compromised, preparing to travel, have vulnerable relatives or considering whether to have a booster jab or not.