Understanding Attomarker SCIENCE
COVID-19 Antibody Immunity Test
Welcome to this expanded explanation of the Attomarker Science. The Attomarker Antibody Immunity Test measures antibodies in the blood to the SARS-CoV-2 coronavirus causing COVID-19. The total IgG response is measured to three antigens on the surface of the virus: Nucleocapsid (N), Spike (S) (and now Omicron Spike) and Receptor Binding Domain (RBD). RBD is the region of the spike protein that docks with the ACE2 receptor to enter our cells and indicates neutralising antibodies.
Natural Antibodies
A patient response to the SARS-CoV-2 infection produces all three types of antibodies and to all three proteins: N, S and RBD Triple Positive ,Figure 1, and we can see the production of antibodies at14 days after symptoms for patients in a hospital recovery trial at St Thomas’.
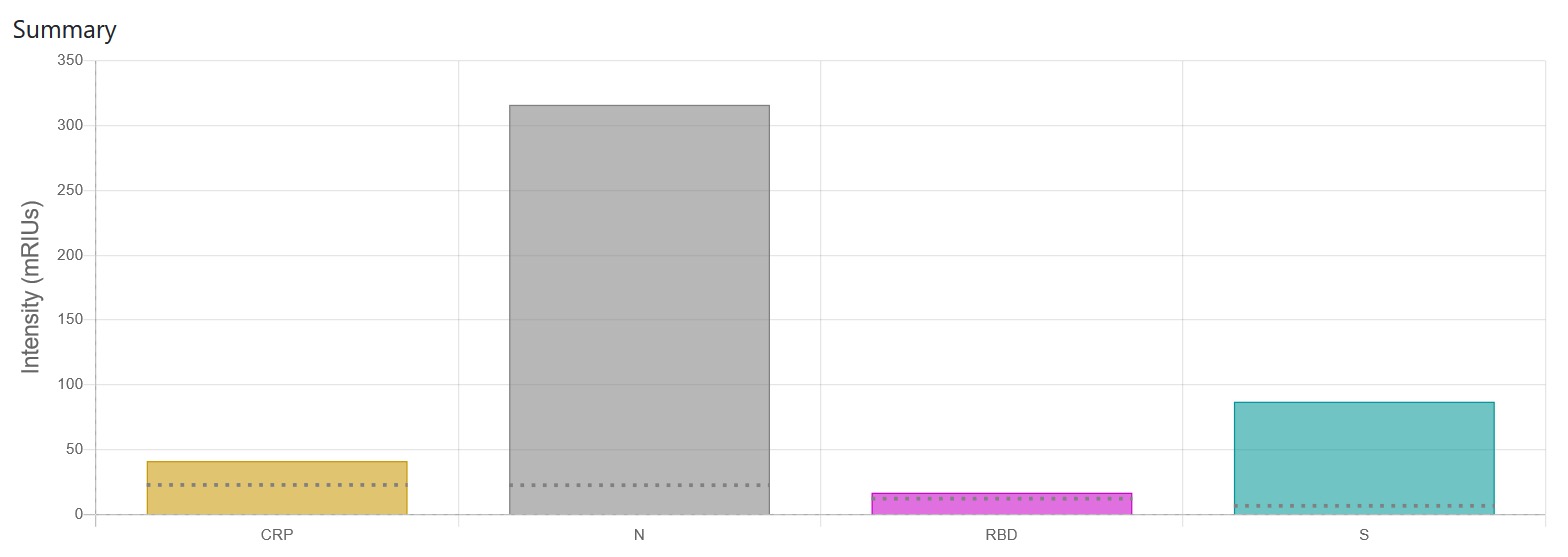
Vaccine Antibodies
The Antibody Immunity Test is also sensitive to antibodies produced by the vaccine characterised by an S and RBD response and not N, such as for the Pfizer vaccine Figure 1. The Test will tell you if the levels of antibodies are above pre-pandemic levels.
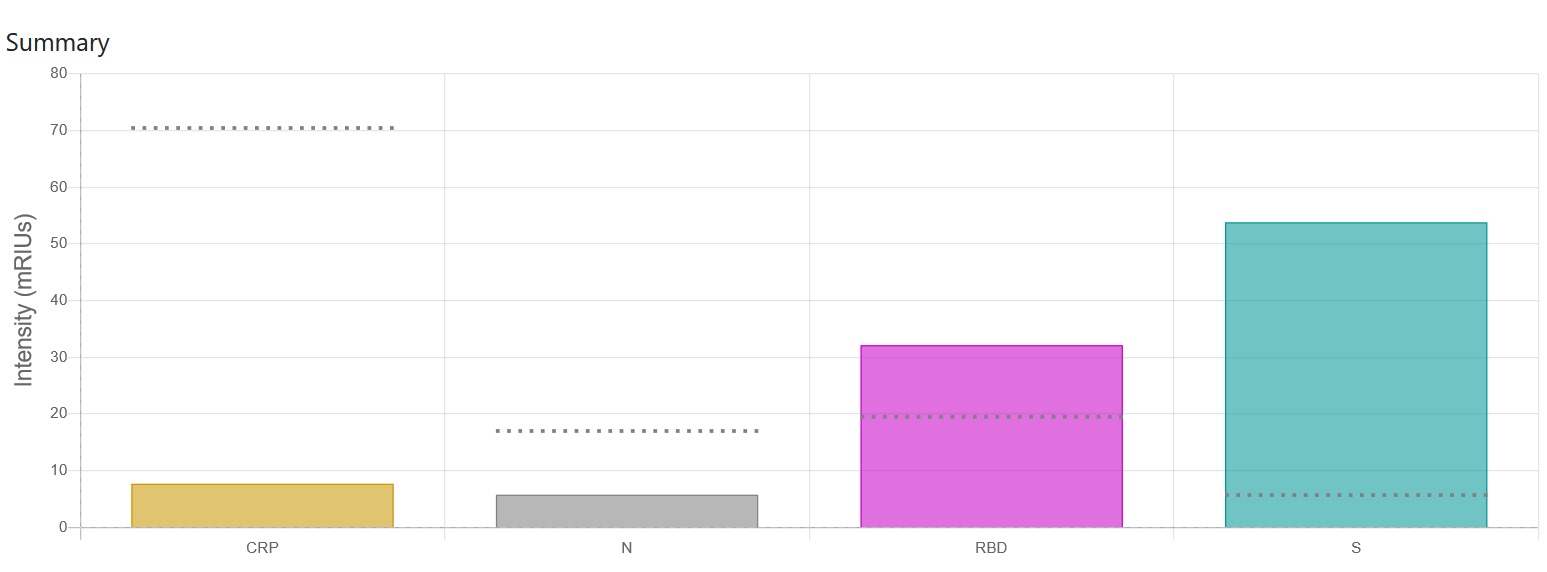
Immunity and Antibody Lifetime
Antibodies to Covid-19 have a half-life. This means that natural antibodies and vaccine-induced antibodies will need to be boosted – a degree of immunity may also be conferred for longer through the T cells and B cells, especially the B Cell “memory” response (immunity and antibody levels may not correlate with T cells). Sufficient levels of antibodies in the blood imply a positive, adaptive immune response which includes the T and B cell response as well as the production of antibodies. The antibody half-life has been measured in an extensive longitudinal study.
Immunity and Antibody Lifetime
Antibodies to Covid-19 have a half-life. This means that natural antibodies and vaccine-induced antibodies will need to be boosted – a degree of immunity may also be conferred for longer through the T cells and B cells, especially the B Cell “memory” response (immunity and antibody levels may not correlate with T cells). Sufficient levels of antibodies in the blood imply a positive, adaptive immune response which includes the T and B cell response as well as the production of antibodies. The antibody half-life has been measured in an extensive longitudinal study.
Long Covid
A significant minority present with “long-Covid” and in a UK survey conducted by ONS around 1 in 10 respondents reported symptoms 12 weeks or longer. It is currently estimated that the UK has 1 million people presenting with long-Covid symptoms; many more may be undiagnosed. The societal and health costs of this condition have yet to be fully realized; this potentially undermines any strategy of “living with Covid-19” as the relationship between the severity of Covid-19 and the contraction of long-Covid is not uniform. It is being reported that a significant number of people, including doctors, have allegedly lost their livelihoods due to long-Covid.
Sensitivity and Specificity
The sensitivity of the Triple Antibody Tests is 96% (93% – 100%) and the specificity is 95% (92% – 99%). This has been validated in a hospital setting for patients admitted with Covid-19 and on collections of commercial samples. The Triple Antibody Test has passed its CE marking validation.
Correlates of Protection
Although Public Health England uses N and S antibody markers and seroprevalence in vaccine surveillance and it is the primary endpoint of the care home surveillance VIVALDI study, currently PHE states there is no agreed correlate of protection with antibody levels. A recent study, yet to be peer reviewed, found 50% protective neutralisation level was estimated to be approximately 20% of the average convalescent level (95% CI = 14-28%). The estimated neutralisation level required for 50% protection from severe infection was significantly lower (3% of the mean convalescent level (CI = 0.7-13%, p = 0.0004). PHE is also carrying out antibody monitoring and is engaged in the Covid-19 Vaccine Surveillance Strategy to assess vaccine efficacy. The Vaccine Surveillance strategy is proposing the differential response for N and S to distinguish between natural infection antibodies and vaccine antibodies and they are using commercially available platforms: Abbott assay targeting N protein (SARS-CoV-2 IgG chemiluminescent microparticle immunoassay (CMIA)) and the Euroimmun assay targeting the S protein (Anti-SARS-CoV-2 ELISA IgG).
- SIREN Study early results – Public Health England published a report looking at the effectiveness of the Pfizer/BioNTech vaccine rollout in England. Their findings suggest that the risk of needing hospital treatment for or dying from Covid-19 was reduced by 75%.
- The case for monitoring changes in antibody levels as a correlate for protection is strong and evidence is continuing to grow for the Covid-19 vaccines and in Government trials.
Long Covid
A significant minority present with “long-Covid” and in a UK survey conducted by ONS around 1 in 10 respondents reported symptoms 12 weeks or longer. It is currently estimated that the UK has 1 million people presenting with long-Covid symptoms; many more may be undiagnosed. The societal and health costs of this condition have yet to be fully realized; this potentially undermines any strategy of “living with Covid-19” as the relationship between the severity of Covid-19 and the contraction of long-Covid is not uniform. It is being reported that a significant number of people, including doctors, have allegedly lost their livelihoods due to long-Covid.
Sensitivity and Specificity
The sensitivity of the Triple Antibody Tests is 96% (93% – 100%) and the specificity is 95% (92% – 99%). This has been validated in a hospital setting for patients admitted with Covid-19 and on collections of commercial samples. The Triple Antibody Test has passed its CE marking validation.
Correlates of Protection
Although Public Health England uses N and S antibody markers and seroprevalence in vaccine surveillance and it is the primary endpoint of the care home surveillance VIVALDI study, currently PHE states there is no agreed correlate of protection with antibody levels. A recent study, yet to be peer reviewed, found 50% protective neutralisation level was estimated to be approximately 20% of the average convalescent level (95% CI = 14-28%). The estimated neutralisation level required for 50% protection from severe infection was significantly lower (3% of the mean convalescent level (CI = 0.7-13%, p = 0.0004). PHE is also carrying out antibody monitoring and is engaged in the Covid-19 Vaccine Surveillance Strategy to assess vaccine efficacy. The Vaccine Surveillance strategy is proposing the differential response for N and S to distinguish between natural infection antibodies and vaccine antibodies and they are using commercially available platforms: Abbott assay targeting N protein (SARS-CoV-2 IgG chemiluminescent microparticle immunoassay (CMIA)) and the Euroimmun assay targeting the S protein (Anti-SARS-CoV-2 ELISA IgG).
- SIREN Study early results – Public Health England published a report looking at the effectiveness of the Pfizer/BioNTech vaccine rollout in England. Their findings suggest that the risk of needing hospital treatment for or dying from Covid-19 was reduced by 75%.
- The case for monitoring changes in antibody levels as a correlate for protection is strong and evidence is continuing to grow for the Covid-19 vaccines and in Government trials.