Last week’s announcement that the most powerful man in the world had contracted COVID-19 calls into question the effectiveness of the White House testing regime.
Screening with a test upon entry to the Rose Garden ‘super-spreader’ event on Sep 26th was clearly used as an excuse to eschew mask-wearing and social distancing, likely based on an inflated test sensitivity figure (the likelihood a ‘negative’ result is correct) that falsely put attendee’s minds at ease.
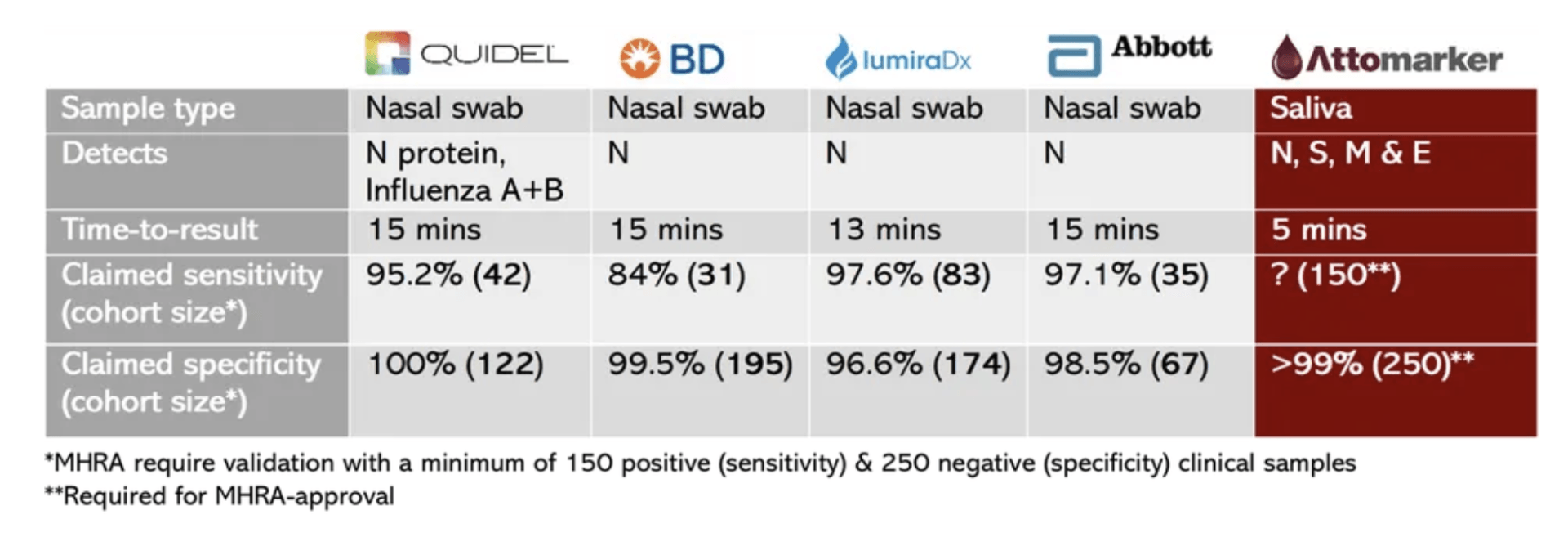
Whilst there has been no official confirmation of the test employed at the event, the Abbott BinaxNOW antigen card is known to be used in the Oval Office. The test, which is FDA Emergency Use-authorised (EUA), claims 97.1% sensitivity and 98.5% specificity (the likelihood a ‘positive’ result is correct), and Abbott even recommend it to be utilised in the manner it was in the Rose Garden, pairing it with a digital “boarding pass” app.
FDA approval is often assumed as a gold standard for diagnostics, however the pandemic has exposed its limitations. The BinaxNOW gained approval and its sensitivity figure based on a cohort of just 35 COVID-positive patients. That means the results of 35 people are now being used to predict whether 50 million people per month have the infection.
For comparison, the UK Medicines and Healthcare products Regulatory Agency (MHRA) will not verify an antigen test validated on fewer than 150 positive and 250 negative samples. In fact, none of the five FDA EUA-approved antigen tests meet these criteria, perhaps the reason that no antigen tests are currently UK-approved. As members of the public are tested, there should be full disclosure of the test used and its limitations.
Attomarker has been committed to scientific rigour in development of its COVID-19 tests since the beginning of the pandemic, and this has not changed with its new saliva-based antigen/antibody test.
We will continue to work closely with government agencies to ensure compliance to stringent standards and deliver a reliable test for mass public use.
Sources
Inside the Flawed White House Testing Scheme That Did Not Protect Trump: https://bit.ly/3nzXvEs
Abbott BinaxNOW™ COVID-19 Ag CARD: https://bit.ly/3jNnfuT
MHRA Target Product Profile: Point of Care SARS-CoV-2 Detection Tests: https://bit.ly/2FeahY2
Quidel Sofia 2 Flu + SARS FIA: https://bit.ly/3jNnlTh
BD Veritor System for Rapid Detection of SARS-CoV-2: https://bit.ly/2SDzq1g
LumiraDx SARS-CoV-2 Ag Test: https://bit.ly/2SHu85a
