Attomarker technology is a label-free particle plasmon resonance multiplexed array that can measure the concentration quantitatively for up to 20 species in 10 µL blood sample in 7 minutes. The original idea came from a proposal to the Engineering and Physical Sciences Research Council of the UK to profile patient blood rapidly, not just for a single marker but for a panel of markers allowing the person’s clinical condition to be treated as a system evolving in time.
Time is the critical variable; time course analysis is possible from the low sampling burden of 10 µL allowing trends to be analysed, rather than single snap-shots of a patient’s health. Attomarker’s vision and that of Prof Shaw’s research laboratory is to profile an individual as a unique endotype; a complex system evolving in time and provide high-quality data rapidly to the clinician to deliver accurate diagnosis. A high-data density, big data, can then be collected, with appropriate permissions, about health trends in populations from which better public health through early diagnosis and monitoring becomes possible.
The technology is multiplex and label-free, as well as quantitative, to enable rapid processing; it’s grounded in the mass sensitivity of localised gold nanoparticle plasmons. Gold nanoparticles (and surfaces) have free electrons which, when excited optically, produce a wave that is localised to the particle but sensitive to the mass just above the surface in the plasmon field, within about 90 nm.
Attomarker technology is an array of gold nanoparticles illuminated in total-internal-reflection and monitored in real time by a video camera, Figure 1. The spots are functionalised with proteins or antibodies specific that provide the specificity for the target assays, as well as a set of control spots and calibrations to ensure a quantitative result.
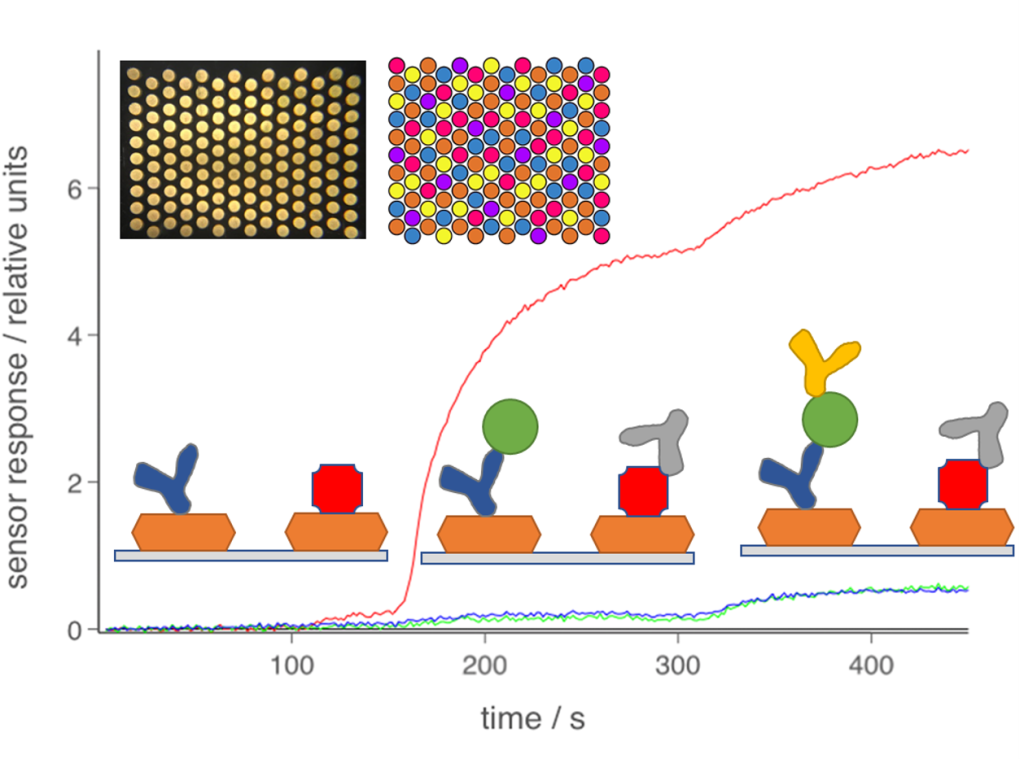
Figure 1 The Attomarker Technology is based on an array of gold nanoparticles (top left) functionalised with antigen, antibodies controls assays. The scatted light is monitored in real time to produce a kinetic trace that through a loading step and calibration step of a sandwich assay leading to a quantitative result.
Each nanoparticle in the array spot contains 300 attograms (10-18 g – a billionth or a billionth of a gram) of biomarker, hence atto+marker. The scattered light is monitored in real-time to produce an immune-kinetic assay observing as a sandwich assay is completed. The evolution of the technology has been published in many papers and has eight granted patents (see below).
The biomarker panel approach is very flexible and can evolve rapidly no more so than in the response ot the SARS-COV-2 pandemic. Attomarker and the Research Group following an appeal for funding to alumni and supporters of Exeter University, decided to demonstrate antibody production on patients suffering from COVID-19 symptoms. We bought five recombinant proteins that had just been made commercially available and combined this into an antibody test with Protein A/G (PAG)a total antibody assay and the acute inflammation marker, C-reactive protein (CRP).
St Thomas’ Hospital, London
We printed the gold arrays – first time with minimal validation because there were no available antibody standards and went to start a collaboration with St Thomas’ Hospital on the 23rd March 2020. We arrived in a deserted London, set up the technology and at 15:16 on the first afternoon, the patient response in below was observed – first time, Figure 2.
The results show the array is working with a strong response from the PAG channel, a huge CRP response over 300 mg/L, a very large response from BSA one of the control channels and then antibodies from Nucleocapsid (N), Spike 1 and Spike 2 proteins. Immediately, we knew the patients were highly inflamed (as did the clinical chemistry laboratory) and that the blood was very viscous from the BSA reference channel.
The viscous blood turned out to be critical with death leading from clotting. Both the CRP and BSA result pointed to the subsequent success of anti-inflammatory and anticoagulant re-purposed drugs in the fight against the disease.
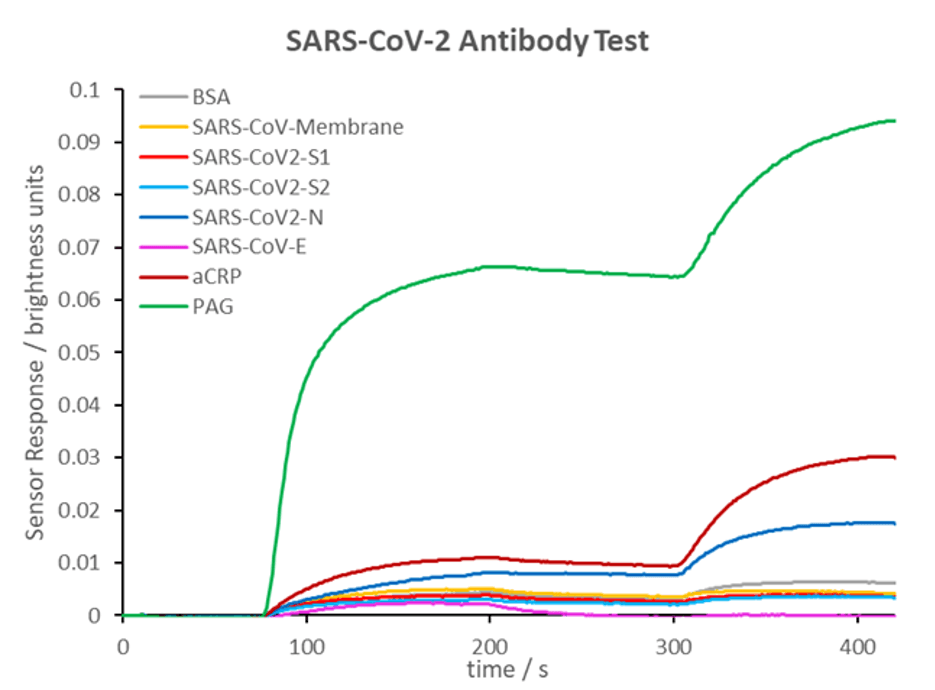
Figure 2 15:16 on 23rd March 2020 St Thomas’ Hospital
The high N response provided an immediate marker for recovery: patients not mounting an immune response to N within about 20 days had a very poor prognosis and ultimately died.
The list of co-morbidities pointing to the high-risk groups in COVID-19 returns to the original vision: which individuals, with particular endotypes, showed different responses? Obesity is often correlated with poor outcomes, probably due to the status of the liver. Attomarker is developing assays to profile the liver, not just its fatty or non-fatty status but its functionality – liver endotypes.
We have also discovered the first evidence of different endotypes in the Complement Cascade where patients naturally have different alternative pathway capacity and rates of activation – capacity endotypes – would point to different responses to antibody complexes.
Endotype profiling is clearly part of the future of diagnosis. Tracing the evolution of an endotype pre-clinically with regular profiling, targeting therapy and procedures and ideally early interventions. Panels of biomarkers can provide near-real time data to refine personalised medicine.
