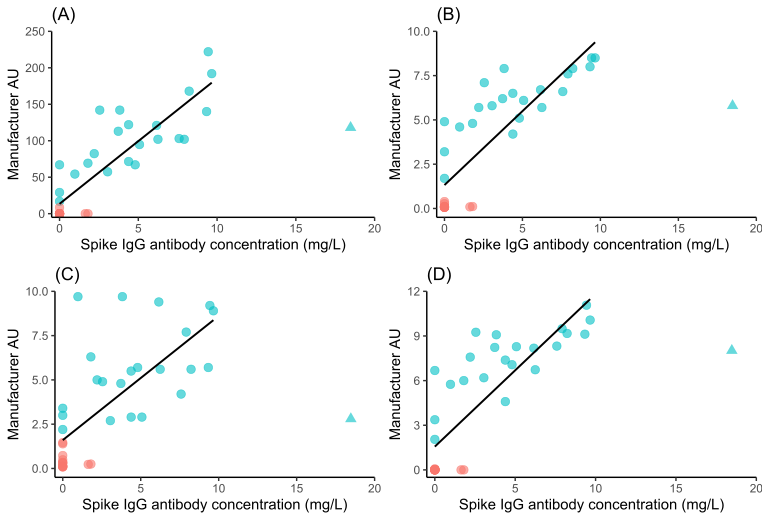
The Attomarker COVID-19 Antibody Immunity Test is a fully quantitative immunoassay calibrated against the conserved S2 regions of the spike protein, omicron spike protein, nucleocapsid and receptor binding domain proteins with recombinant antibodies calibrated against the mass standard material NIST Human Antibody.¹ ² The test has been calibrated against the NIBSC (WHO) reference materials and compared against four other manufacturers for linearity, Figure 1 and Table 1.
Data for all manufacturers and the calibrations are being collated and are available on request.
Figure 1: Plots of quantified Spike IgG Antibody result in mg/L vs four external tests for the NIBSC 20/B770 sample panel. Test platform (A) measures S1/S2 IgG; platform B ELISA S1 (recombinant) IgG; platform C ELISA S1 IgA and platform D S1 IgG. Sample with NIBSC sample ID 19 has been excluded from the fit. Pink circles are NIBSC negatives samples; Blue circles are positive NIBSC samples. The point depicted as a triangle is an outlier despite several repeats and was removed from the fit.
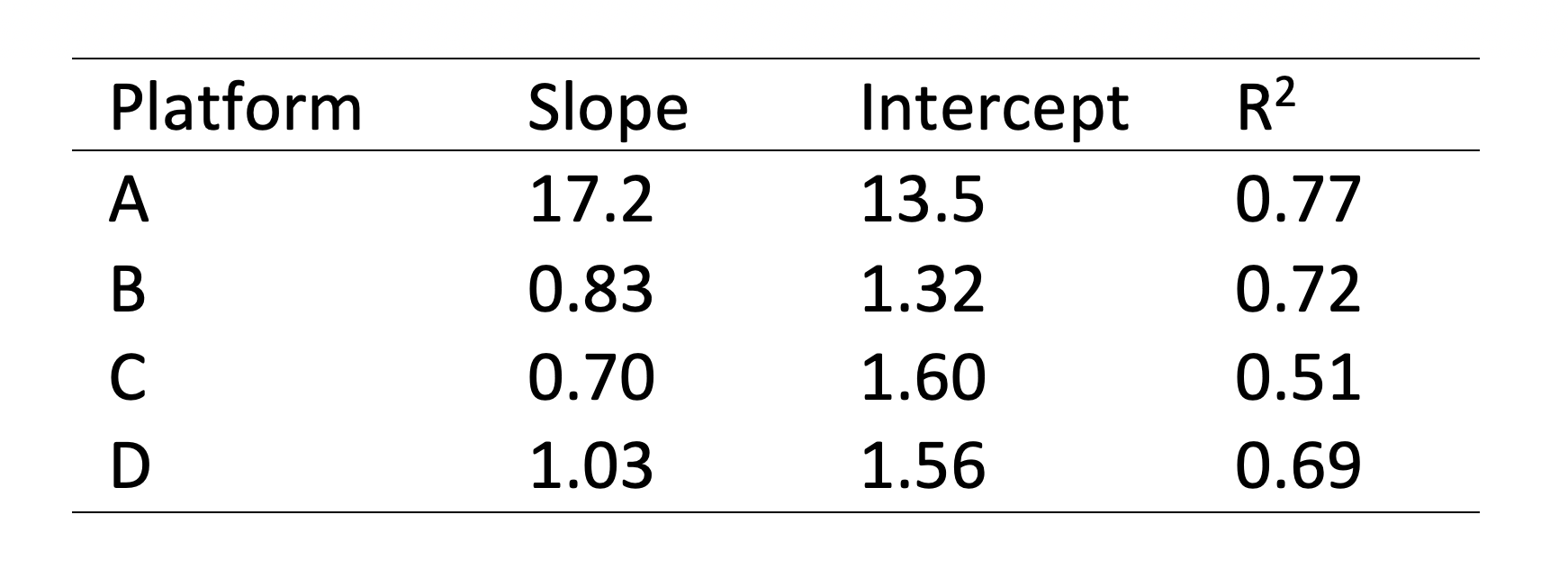
Table 1: Lines of best fit and Correlation coefficients for the comparison with four platform technologies against the current fully quantitative test.
Important of SI Units – mg/L
The importance of standardised assays has been recognised by many ³ providing immediate comparison between cohorts and studies and in the case of Attomarker all against a standard material in the NIST Human Antibody. The NIST mAb is a recombinant humanized IgG1ĸ with a known sequence ⁴ specific to the respiratory syncytial virus protein F (RSVF). ⁵
Expressing assay results in an SI format immediately provides comparisons between assays from different manufacturers. Scientifically, the role of an SI unit immediately points to understanding and mechanisms. As an example, the UK Government Panel Treatment of immuno-compromised patients with pre-exposure prophylaxis with tixagevimab 300 mg plus cilgavimab 300 mg (Evusheld) would be expected to correspond to a peak concentration of ~200 mg/L, instantly measurable and comparable between hospitals.
Conclusions:
- COVID-19 antibody test can now be standardised internationally against a fully characterised international standard material, the NIST Human Antibody.
- In the future all immuno-sandwich assays can be calibrated against this standard, unifying the field.
References:
- James-Pemberton PH, Helliwell MW, Olkhov RV, et al. Fully Quantitative Measurements of the Antibody Levels for SARS-CoV-2 Infections and Vaccinations calibrated against the NISTmAb Standard IgG Antibody. medRxiv 2022: 2022.07.12.22277533.
- James-Pemberton PH, Helliwell MW, Olkhov RV, et al. Vaccine, Booster and Natural Antibody Binding to SARS-CoV-2 Omicron (BA.1) Spike Protein and Vaccine Efficacy. medRxiv 2022: 2022.07.12.22277539.
- Cantoni D, Mayora-Neto M, Nadesalingam A, et al. Neutralisation Hierarchy of SARS-CoV-2 Variants of Concern Using Standardised, Quantitative Neutralisation Assays Reveals a Correlation With Disease Severity; Towards Deciphering Protective Antibody Thresholds. Front Immunol 2022; 13: 773982.
- Formolo T LM, Levy M, Kilpatrick L, Lute S, Phinney K, et al. . Determination of the NISTmAb primary structure. State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization: ACS Symposium Series; 2015: 1-62.
- McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nature structural & molecular biology 2010; 17(2): 248-50.
