The Immune Response to SARS-CoV-2 and Long COVID
Long COVID is currently reported to affect as many as one in five individuals who have had a COVID infection in the UK, with each new SARS-CoV-2 infection adding to the risk burden of developing the debilitating Infection Associated Chronic Condition (IACC). This condition is associated with significant increases in long-term ill health and work absence. The finding that multiple infections increases vulnerability to long COVID (Statistics OoN, 2021) indicates that measures to protect the population are highly desirable.
To fully eliminate an infection caused by SARS-CoV-2 – the virus responsible for COVID-19 – the body tends to rely on two key parts of the immune system: antibodies and T cells, a special type of white blood cell. These immune responses are essential for clearing the virus from its initial infection site, which is typically a localised area in the body where the virus has not yet spread.
For the virus to be completely removed from the entire body (a process called systemic clearance), the immune system needs what is called a sterilising serum – a state where antibodies are able to bind to the virus and neutralise it through complexation. This is where the virus and antibodies form a stable complex that can then be cleared away.
If the virus is not fully cleared, it is thought it may remain in the body in small amounts. This hypothesised viral persistence has been linked to long COVID, where symptoms continue or appear after the initial infection has passed. People with long COVID often experience a range of symptoms, including brain fog, headaches, fatigue, muscle pain, problems with breathing or heart function, and digestive issues (Yong, 2021).
Endotype-Driven Medicine and Personalised Healthcare
In an Attomarker study, researchers identified several distinct types of immune responses (known as antibody immunity endotypes) which are linked to how people’s antibodies respond to different Spike protein variants of the SARS-CoV-2 virus. These endotypes differ not only in the quantity of antibodies produced but also in their effectiveness (as measured by avidity or “stickiness”).
This approach, sometimes called endotype-driven medicine by clinicians and scientists, focuses on identifying specific biological and chemical patterns that help explain why different people experience different outcomes after infection. It represents a promising direction for the future of personalised healthcare.
Attomarker research has found one particularly strong immune response, referred to as the Universal Endotype (U(+)), in about 25% of individuals tests. People with this endotype produce significant amounts of high-quality antibodies that respond well to all variants of the virus tested. However, the remaining 75% of individuals show variances in their antibody production and avidity, which may leave them more vulnerable to ongoing infection. These endotype gaps could potentially allow the virus to remain in the body and may be linked to the development of long COVID (James-Pemberton et al., 2022).
The Role of Attomarker
Attomarker is pioneering precise instruments and assays that measure the immune response to SARS-CoV-2. Their innovative COVID Antibody Spectrum Test helps identify endotype gaps in immunity, which is crucial for understanding and addressing long COVID. An antibody immunity spectrum is the range of antibodies in the body, including their quantity, quality, diversity, and durability, that help protect against infections. Attomarker’s recent Antibody research has shown that people with long COVID appear to fall into three groups:
- Approximately 60-65% of long COVID patients fall into a category where their immune response is compromised.
- Around 10-15% of others show signs of a hyper-immune reaction.
- The remainder do not have a readily classifiable spectrum at the time of testing; it is hoped that sufficient datasets and advanced pattern analysis will enable further interrogation of this cohort.
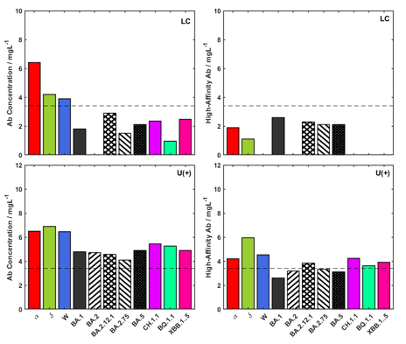
Figure 1 Immunity Endotype for long COVID patient reporting onset of symptoms associated with a Wuhan infection, Long COVID (LC), and the Universal Endotype response, U(+).
It is argued that this initial classification enables clinicians to engage with more personalised therapeutic strategies based on a patient’s specific immune profile, which should constitute a significant step towards more effective treatments. To illustrate, the antibody spectrum of a U(+) patient and a long COVID (LC) patient can be seen in Figure 1. The LC patient has an endotype with a notable gap in antibody quality, in this case, to the Wuhan variant, and later Omicron sub-variants. The endotype gap for the Wuhan variant in particular, correlates with the onset of their long COVID symptoms. Attomarker’s novel diagnostic-to-therapeutic approach could help address these gaps by working with doctors and using the right treatments based on test results and available therapies for different virus variants.
Therapeutic Approaches
Following an Attomarker test, a primary care provider specialising in Long COVID chose a treatment for a patient presenting with an immune gap associated with the Wuhan variant. Specifically, an immunotherapy drug that targets that variant.
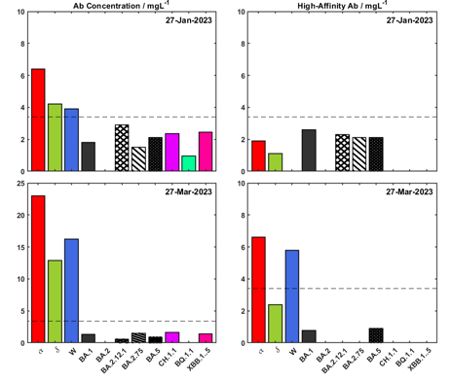
Figure 2 Long COVID Endotype. Test results on 27th January 2023 and 27th March 2023 (following the first dose of the drug), showing a filled gap in the immunity spectrum for the Wuhan variant.
For instance, the patient in Figure 2 received a single dose of Evusheld, which improved their immune response right away. The treatment is given as an injection into the muscle and works gradually. Encouragingly, this patient reports changes in prevalent symptoms, such as decreased frequency of migraines and improved gut performance; however, it is still relatively early in the treatment cycle.
After one or two half-lives of the therapy (the time it takes for half of a drug to be eliminated from the body), the antibody concentration will resemble the vaccine response. At this point, a hetero dose of a vaccine (one not previously seen by the patient), such as Novavax, may be given to break the immunity imprint and further repair the gap in the immunity spectrum.
The Future of Long COVID Treatment: Precision Medicine and Next Steps
The next step is to collaborate with leading clinics to launch a prospective cohort study, potentially using AstraZeneca’s monoclonal antibody, Sipavibart (Kavigale). This therapy has already been approved in the EU and is under review by the MHRA, showing potential in early intervention. Moving forward, Attomarker is leading a fundraising effort to fund the first patients through this innovative treatment, which will provide critical data on the real-world effectiveness of monoclonal antibody therapy for long COVID patients.
Attomarker is excited about the potential of using advanced diagnostics to determine individually tailored therapeutics for long COVID and, in the future, a range of other diseases and conditions. This personalised approach to treatment is a major step forward in healthcare, allowing for precision medicine that caters to each patient’s unique biological and immune characteristics.
Attomarker will continue to report progress and share insights as our clinical trials and studies unfold. To sign up for notifications go to: https://attomarker.com/support/register/
References
James-Pemberton, P.H., Kohli, S., Westlake, A.C., Antill, A., Olkhov, R.V. and Shaw, A.M., 2022. Antibody Affinity Maturation to SARS-CoV-2 Omicron Variants in a Teachers Cohort. medRxiv. Available at: https://www.medrxiv.org/content/10.1101/2022.12.01.22282932 [Accessed 10 Apr. 2025].
Khoury, D.S., Cromer, D., Reynaldi, A., et al., 2021. What level of neutralizing antibody protects from COVID-19? medRxiv. Available at: https://www.medrxiv.org/content/10.1101/2021.03.09.21252641 [Accessed 10 Apr. 2025].
Statistics OoN., 2021. The prevalence of long COVID symptoms and COVID-19 complications. Office for National Statistics. Available at: https://www.ons.gov.uk [Accessed 28 Apr. 2021].
Yong, S.J., 2021. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infectious Diseases, 53(10), pp.737-754.
